Difference between revisions of "Neuroscience"
| Line 1: | Line 1: | ||
[https://www.youtube.com/watch?v=Wj3C6cLqXzY Axon Growing Video] | [https://www.youtube.com/watch?v=Wj3C6cLqXzY Axon Growing Video] | ||
| − | :When and NGF | + | :When and NGF bead lands on an axon, a new growth cone is induced at the contact site, and a branch sprouts from the axon. |
| + | |||
| + | From [http://growthcones.neuroscience.umn.edu/Welcome.html Neuronal Growth Cones] | ||
---- | ---- | ||
Revision as of 18:09, 10 September 2014
- When and NGF bead lands on an axon, a new growth cone is induced at the contact site, and a branch sprouts from the axon.
(2014) Regulatory mechanisms underlying the differential growth of dendrites and axons
Media:2014_--_Regulatory_mechanisms_underlying_the_differential_growth_of_dendrites_and_axons.pdf
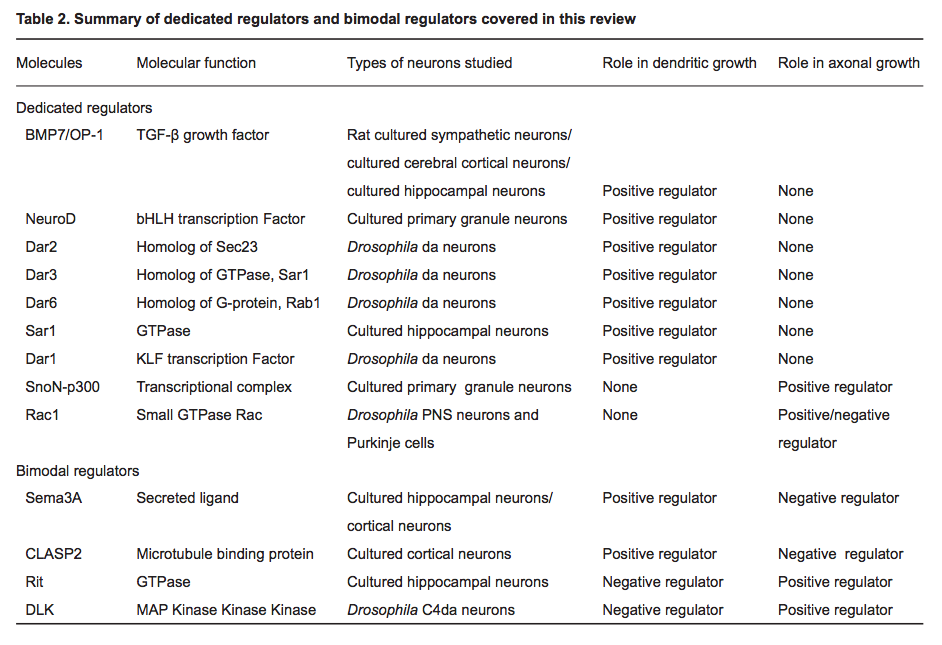
- "Recent studies have uncovered two distinct types of regulatory mechanisms that differentiate dendritic and axonal growth: dedicated mechanisms and bimodal mechanisms. Dedicated mechanisms regulate either dendrite- specific or axon-specific growth; in contrast, bimodal mechanisms direct dendritic and axonal development in opposite manners."
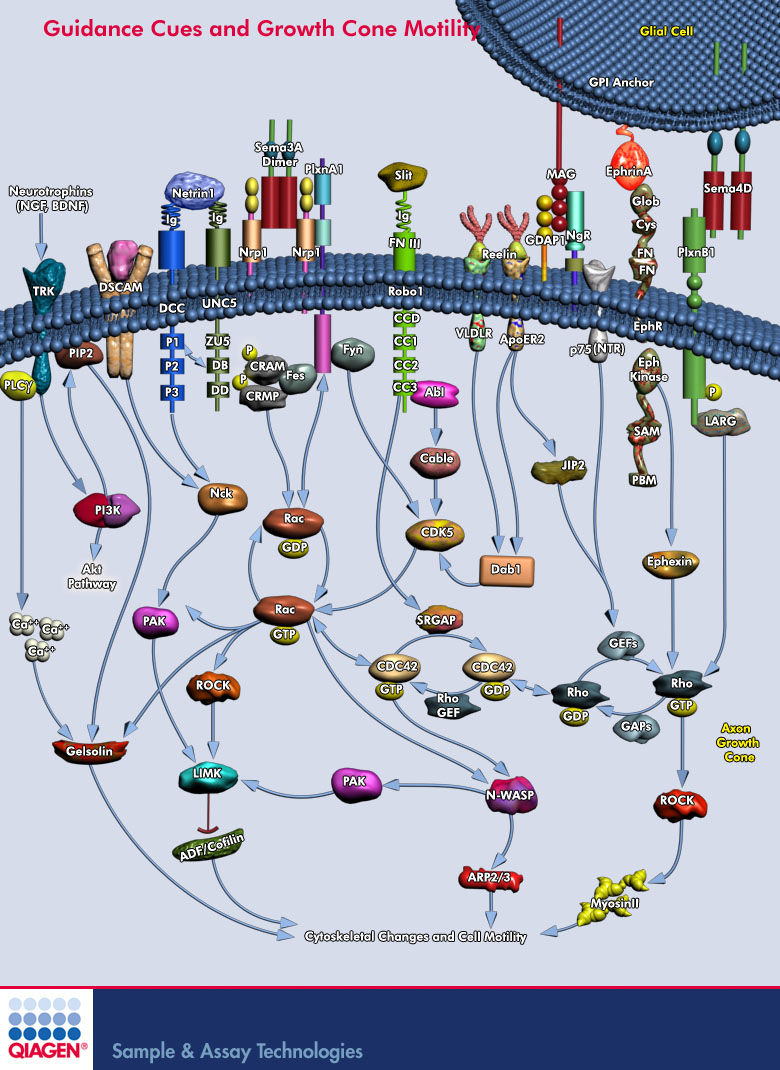
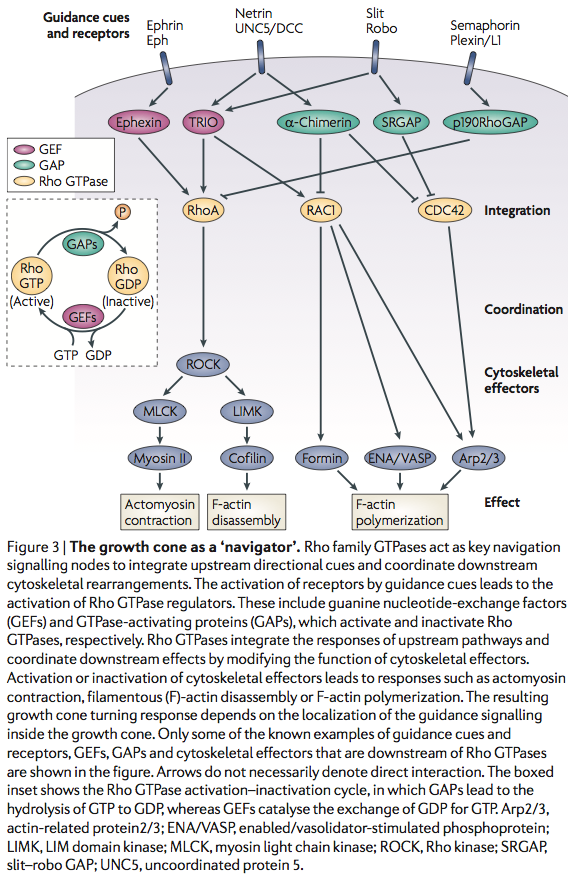
Sema3A is where?
Sema3A -> CRMP -> Rac/GDP -> Rac/GTP -> CDC42 -> Rho -> ROCK -> myosinll
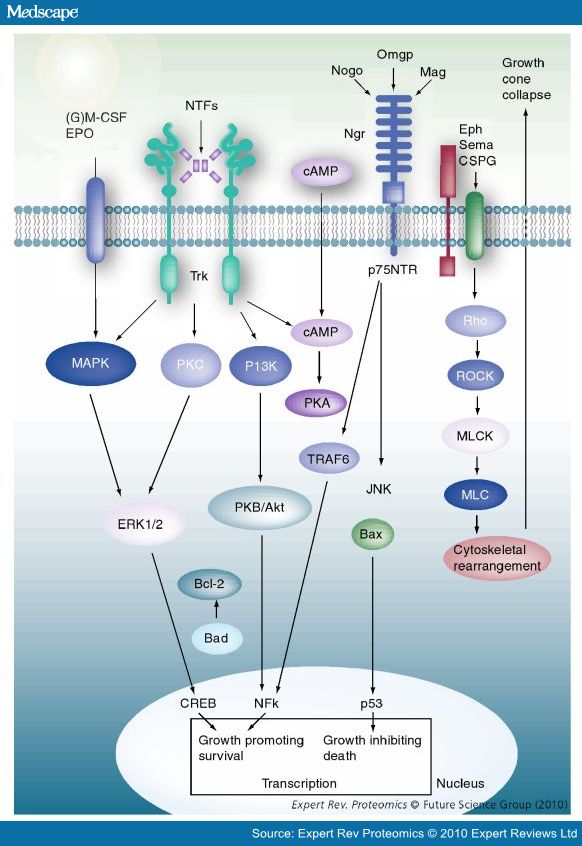
(2010) Deciphering Proteins and their Functions in the Regenerating Retina
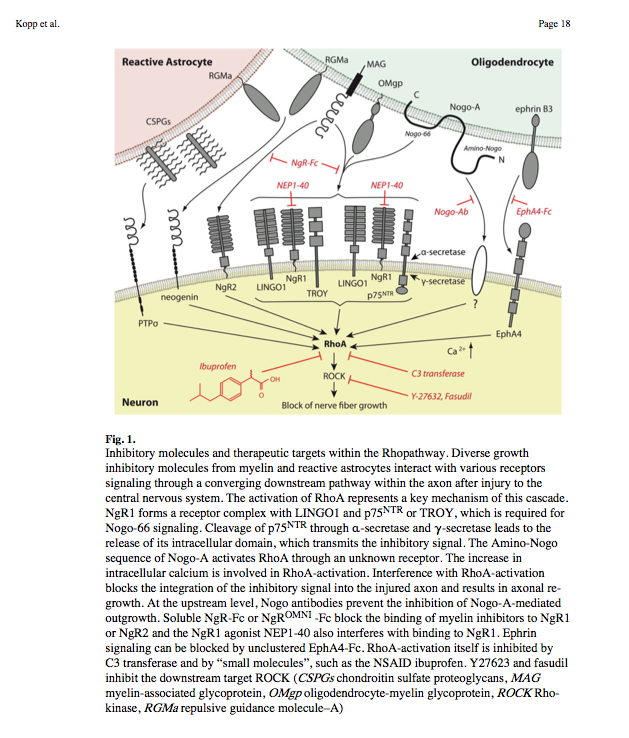
- Nogo-A, -B and -C all contain a 66-amino acid extracellular domain (Nogo-66) that alone can inhibit neurite outgrowth and induce growth-cone collapse.
- Remarkably, a single neuronal protein (NgR), binds Nogo, MAG and OMgp.[101] NgR is thought to form a complex with its coreceptors (p75NTR, TROY, a member of the TNF receptor family, and Lingo-1), thereby transducing a myelin/NgR signal as the inhibitory signal to the cell interior[95] and potentially activating the small GTPase RhoA (Figure 3B). Another myelin-associated neurite outgrowth inhibitor, the RGM, which activates the Rho/ROCK signaling cascade independently of the NgR pathway, has recently been discovered. Consistent with these findings, deleting oligodendrocytes, myelin or Schwann cell-derived factor-induced cleavage of NgR and Nogo-A, and inactivation of p75NTR [105] enhances the regeneration of descending visual pathways. Consistent with their inhibitory effects on neurite outgrowth, approaches counteracting NgR, Nogo-A or RhoA have been shown to significantly enhance long-distance axon regeneration in animal models of acute retinal injury.
^ need to check up on RGM! Looks as though disabling NgR is not sufficient. also need to disable RGM as either one can activate Rho -> ROCK.
So how about directly disable Rho using Ibuprofen:
Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury
(2005) Local translation of RhoA regulates growth cone collapse
- Neuronal development requires highly coordinated regulation of the cytoskeleton within the developing axon. This dynamic regulation manifests itself in axonal branching, turning and pathfinding, presynaptic differentiation, and growth cone collapse and extension. Semaphorin 3A (Sema3A), a secreted guidance cue that primarily functions to repel axons from inappropriate targets, induces cytoskeletal rearrangements that result in growth cone collapse1. These effects require intra-axonal messenger RNA translation. Here we show that transcripts for RhoA, a small guanosine triphosphatase (GTPase) that regulates the actin cytoskeleton, are localized to developing axons and growth cones, and this localization is mediated by an axonal targeting element located in the RhoA 3' untranslated region (UTR). Sema3A induces intra-axonal translation of RhoA mRNA, and this local translation of RhoA is necessary and sufficient for Sema3A-mediated growth cone collapse. These studies indicate that local RhoA translation regulates the neuronal cytoskeleton and identify a new mechanism for the regulation of RhoA signalling.
Intra-Axonal Translation of RhoA Promotes Axon GrowthInhibition by CSPG
- RhoA transcripts have been detected in axons of embryonic dorsal root ganglia (DRG), retinal ganglion, and cortical and hippocampal neurons, and are locally translated in DRG axons in response to the inhibitory guidance cue Semaphorin 3A (Sema3A) and mediate growth cone collapse (Wu et al., 2005).
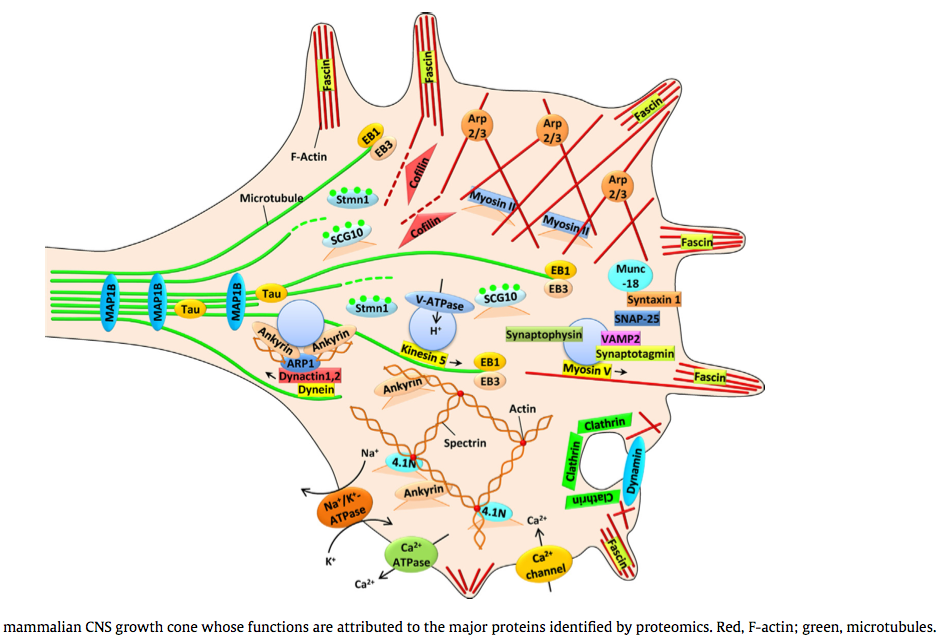
(2014) Proteomic identification of the molecular basis of mammalian CNS growth cones
Media:2014_--_Proteomic_identification_of_the_molecular_basis_of_mammalian_CNS_growth_cones.pdf
The trip of the tip: understanding the growth cone machinery
File:(2009) The trip of the tip -- understanding the growth cone machinery.pdf
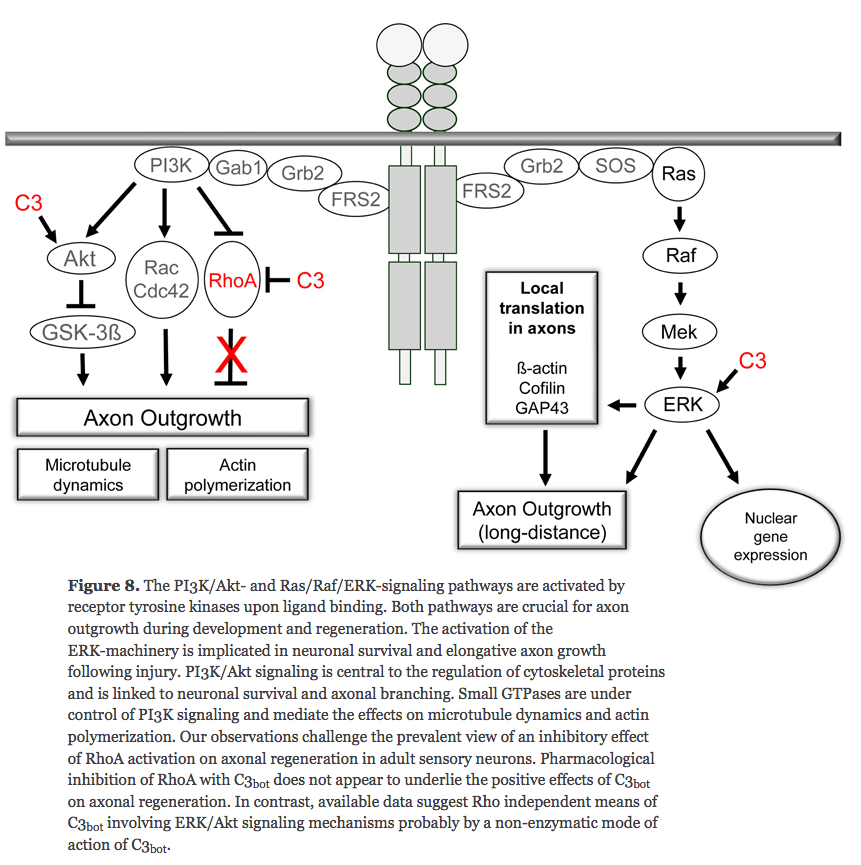
[journal.frontiersin.org/Journal/10.3389/fncel.2012.00043/full Rho-independent stimulation of axon outgrowth and activation of the ERK and Akt signaling pathways by C3 transferase in sensory neurons]
^ looks like disabling RhoA doesn't encourage axon growth outside of the brain
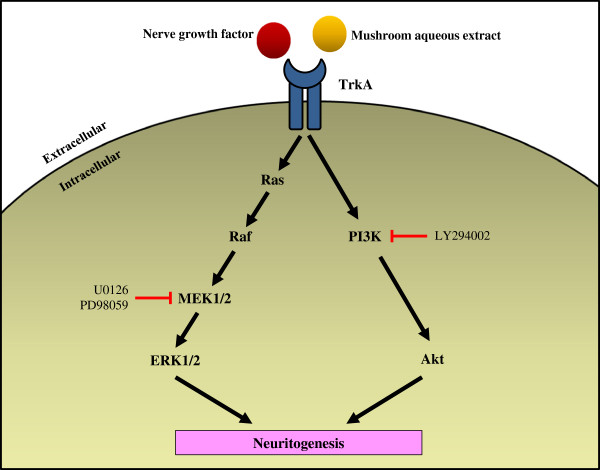
Shrooms
Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells
DIHEXA
Ashwagandha
Axon (or dendrite) predominant outgrowth induced by constituents from Ashwagandha
- "results suggest that axons are predominantly extended by withanolide A, and dendrites by withanosides IVand VI."
Media:2002_--_Axon-_or_dendrite-predominant_outgrowth_induced_by_constituents_from_Ashwagandha.pdf
- withanoside IV a.k.a. Sominone = GDNF-independent stimulator of the RET pathway and/or a novel modulator of RET signalling
[1]